Abstract
Introduction: Although survival rates have improved over the years, chronic graft-versus-host disease (cGvHD) remains the most frequent and severe complication in the long term after allogeneic hematopoietic stem cell transplantation (allo-HSCT). All the strategies developed to reduce its incidence are based on procedures aimed to decrease the risk of acute GvHD that, consequently, can also reduce the risk of cGvHD, mainly using immunosuppression in the early post-transplant period. These strategies induce apoptosis of donor T lymphocytes, responsible for a/cGvHD but also for graft-versus-tumor response. In the present project, we have evaluated a new strategy aimed to reduce the risk of cGvHD by manipulating the immune response not in the early post-transplant period, but in later phases.
cGvHD develops via a complex cellular and molecular network involving thymus damage and unusual antigen presentation leading to aberrant T- and B-cell reactions characterized by Th17/Tc17 differentiation, macrophage sequestration in tissue, alloantibody formation, and fibrosis. Most of these cell populations are dependent on NF-kB for their activation. Ixazomib is a second-generation proteasome inhibitor for oral administration, representing an ideal candidate for prophylaxis in GvHD.
Objective: We propose to develop a murine model of cGVHD and progressive onset cGvHD to test the efficacy of delayed administration of ixazomib as a novel strategy to decrease the risk of cGvHD.
Methods: For in vitro studies, peripheral blood mononuclear cells from healthy donors were stimulated in the presence of different concentrations of ixazomib. After 48h and 120h, apoptosis was analyzed, and activation markers were evaluated.
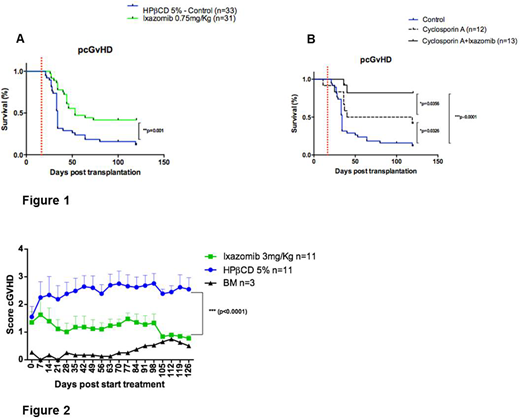
For in vivo studies, a murine model of progressive cGvHD (pcGvHD - allowing the recipient to survive to mild aGvHD, thus favoring autoreactive T cells to expand and cause cGvHD) and a scleroderma model of cGvHD were used. Ixazomib at 0.75mg/Kg/twice weekly, 2X from day +21 in the pcGvHD, with or without cyclosporine A (CyA) at 5mg/Kg/day from day 0 until the end of the study. For scleroderma cGvHD group, 3mg/Kg/2X from day +30 was used up to 120 days post-transplant. Flow cytometry was used to evaluate the different lymphocyte populations in the different target organs of the GvHD.
Results:In vitro results showed that activated T lymphocytes are sensitive to the proapoptotic effect of ixazomib (>100nM), while high concentrations of the drug are necessary to cause apoptosis in the non-activated cells (5000nM). Through CD27 and CD45 expression, we verified that ixazomib has a proapoptotic effect mainly on naïve and effector cells. We also verified a decrease in the activation markers of CD3+CD25+INF-γ+ cells (p˂0.01, 1000nM, n = 4).
In the animal trial, the survival of the pcGvHD model mice treated with ixazomib was significantly higher as compared to untreated mice, with a significant decrease in the signs of pcGvHD (Figure 1A). In addition, the combination of CyA and ixazomib improved survival as compared to those mice receiving CyA or ixazomib alone as well as untreated controls (Figure 1B). In the scleroderma cGvHD model, we observed that the animals treated with ixazomib showed significantly less signs of GvHD (p˂0.0001) (Figure 2).
Multiparametric flow cytometer analyses showed that in the pcGvHD model, mice treated with ixazomib had a decrease of effector CD4 T-cells in bone marrow (p=0.06) and spleen (p=0.015) when compared to untreated mice. Also an increase of CD19+ cells in the colon (p=0.04), liver (p=0.035), lymph nodes (LN) (p=0.037) and lungs (p=0.03) was observed. Regarding the regulatory T cells (Foxp3+), the treated mice had a significant increase in LN (p=0.02), Peyer patches (p=0.015) and thymus (p=0.028) as compared to untreated mice.
Conclusion:In vitro studies show that ixazomib induces apoptosis on activated T lymphocytes and decreases the expression of activation markers. Our in vivo model indicates that the combination of CyA and ixazomib for the prevention of pcGvHD and cGVHD after allogeneic HSCT is promising and merits further investigation in clinical trials.
Figure 1. Survival of pcGvHD.
Figure 2. Score graphic of scleroderma cGvHD. BM - Bone marrow, mice that were transplanted with BM from BALBc mice (Syngeneic).
Ramos:Takeda Oncology: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.